Research Projects
Hepatocellular Carcinoma
From December 23, 2021, to August 18, 2024, a multicenter clinical trial was conducted across seven Chinese institutions: Beijing You'an Hospital affiliated to Capital Medical University, Renji Hospital affiliated to Shanghai Jiao Tong University School of Medicine, Henan Cancer Hospital, and four additional centers. A total of 180 patients were enrolled, with the experimental group receiving irreversible electroporation ablation and the control group undergoing radiofrequency ablation.
Inclusion Criteria: Age 18 to 80 years (both sexes);Solitary tumor ≤4 cm in diameter;OR multiple tumors (≤3 lesions) with maximum diameter ≤3 cm;Life expectancy >6 months.
RESULTS:At 1, 3, and 6 months postoperatively, the lower limits of the 95% confidence intervals (CIs) for the difference in ablation success rates between the experimental and control groups exceeded the prespecified non-inferiority margin. Thus, the Compound Steep Pulse Therapy technique provided safe and clinically effective treatment for hepatic malignancies in this study.
| Ablation at 1 Month Technical Success Rate | Ablation at 3 Month Technical Success Rate | Ablation at 6 Month Technical Success Rate | |
|---|---|---|---|
| Experimental Group | 97.67% | 95.24% | 93.98% |
| Control Group | 93.98% | 93.90% | 88.61% |
| Pre-ablation | 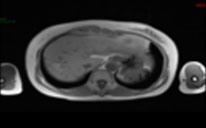 |  |  |
| Post-ablation |  |  |  |



