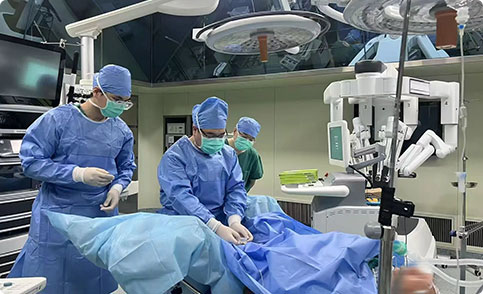
Research Projects
Prostatic Hyperplasia
From August 2020 to July 2022, a multi-center clinical trial was conducted across nine hospitals: Renji Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Qilu Hospital of Shandong University, Tongji Hospital Affiliated to Tongji Medical College of Huazhong University of Science and Technology, Hunan Provincial People's Hospital, Shanghai Pudong Hospital, Ningbo Huamei Hospital Affiliated to University of Chinese Academy of Sciences, Yiyuan County People's Hospital, Shanghai East Hospital Affiliated to Tongji University, and Sun Yat-sen Memorial Hospital of Sun Yat-sen University. A total of 160 patients with benign prostatic hyperplasia (BPH) were enrolled, with 80 cases assigned to the experimental group (treated with compound steep pulse therapy) and 80 cases to the control group (treated with tamsulosin hydrochloride extended-release capsules). All patients underwent a 3-month follow-up period.
Inclusion Criteria:(1) Age 50 to 80 years; (2) International Prostate Symptom Score (IPSS) ≥ 12 points; (3) Maximum urinary flow rate (Qmax) > 5 mL/s and ≤ 15 mL/s, etc.
RESULTS:The surgical group showed significantly greater improvement in urinary flow rate (Qmax) versus medication controls (p<0.01), with zero severe adverse events, mean operative time ~15 minutes, and same-day ambulation due to minimal invasiveness.