NORTION® Composite Steep Pulse Therapy Device Model HFMP-01E CE Approved for Market Launch NORTION® PCa & NORTION® BPH Domestically Approved in China
Following last month's achievement of the FDA Breakthrough Device Designation (BDD) for its independently developed Composite Steep Pulse Therapy Device, NORTION has now achieved major innovative breakthroughs on October 24!
On October 24, NORTION's independently developed Composite Steep Pulse Therapy Device secured CE certification approval for market launch, obtaining the world's first CE certification for High-Frequency Pulsed Electric Field (H-FPEF) Ablation Technology specifically for prostate cancer treatment.
On the same day, NORTION's Portable Prostate Cancer & BPH Therapy Device received Class III Certification from China's National Medical Products Administration (NMPA), paving the way for deploying pulsed electric field ablation technology in primary care settings.
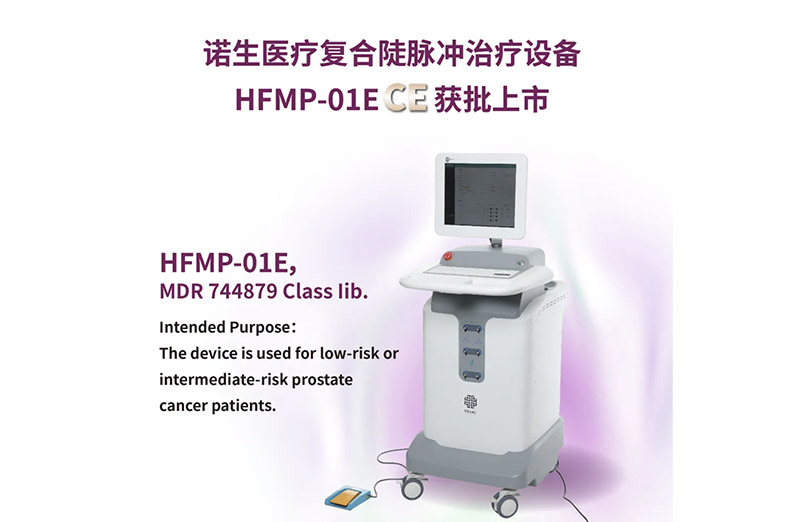


With the NORTION Composite Steep Pulsed Therapy Device receiving the U.S. FDA "Breakthrough Device Designation" and subsequent CE Mark approval in the EU for its prostate cancer indication, this marks not only a new breakthrough in NuoSheng Medical's global strategic layout, but also a significant milestone for China's domestic pulsed field ablation industry in advancing onto the global stage. NuoSheng Medical will seize this opportunity to continuously expand its overseas presence, aiming to meet global clinical needs through high-quality pulsed ablation treatment solutions.



